Client
Omi Home Furnishings Manufacturing
Services
Digital Audit, Market Research, User Experience
Year
2022
The difficulty level of a subject depends on a student’s strengths, passions, and capabilities. A neat way to find out if A-Level Chemistry will be hard for you is to ask a simple question. “Was GCSE hard for me?” If it was, then A-Level would be way more difficult. Since it’s a huge step up from GCSE.
But suppose you want to take Chemistry at the university level. In that case, you’ll need to be able to excel and enjoy Chemistry at the A-Level!
To give you a headstart on how hard chemistry may be, let’s look at how many students managed to score A*s and As, then compare the data with other subjects. According to Ofqual Analytics, here are the A-Level 2021 outcomes for Chemistry, Maths,
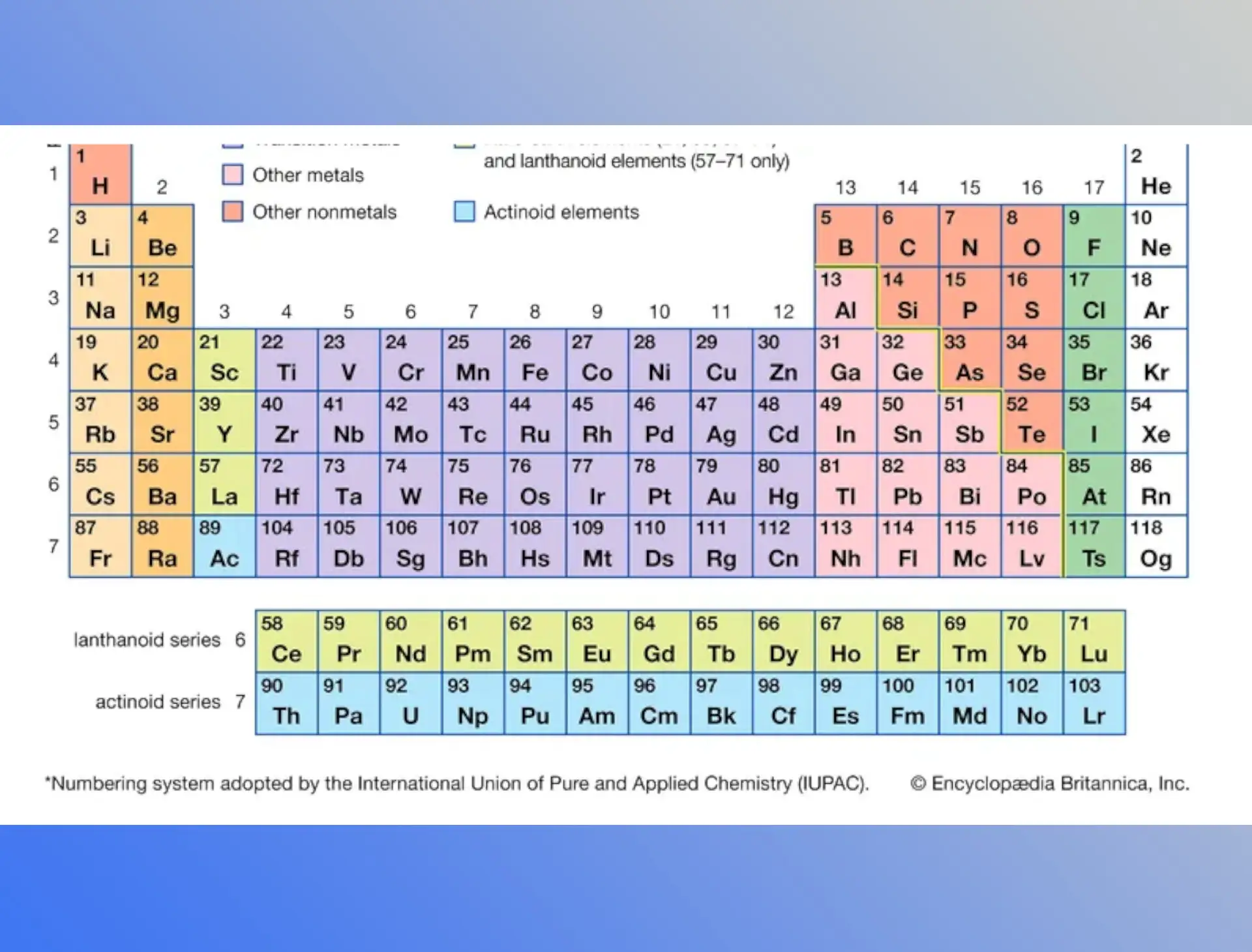
Chemical periodicity has been described as the characteristics of elements showing a pattern and recurring variation with increasing atomic number. In the beginning, just 31 elements were known, but currently, 118 elements are known. As a result, studying the chemistry of all the different elements is extremely challenging. Scientists are looking for a systematic technique to rearrange and organize knowledge by categorizing it in order to solve this dilemma.
The Origins of Periodic Classification
Dobereiner’s Triads: In 1817, Johann Wolfgang Dobereiner, a German scientist, attempted to organize substances having similar characteristics into groups. He demonstrated that three elements might be arranged in a triad so that the atomic mass of the middle element is about equal to the average of the atomic masses of the other two elements. As a result, these groupings are known as ‘triads.’ For example, Li has an atomic mass of 6.94, while K has an atomic mass of 39.10. Na, the middle element in this triangle, has an atomic mass of 22.99.
The disadvantages of Dobereiner’s Triads were that these were inapplicable to elements with extremely high and low atomic masses, and this categorization was not applicable to all of the elements.
The Law of Octaves by Newland: In 1866, John Newlands, an English chemist, organized the 56 known elements in order of increasing atomic mass, beginning with hydrogen and ending with thorium. He found a pattern in every eighth element that had qualities identical to the first one. He linked these parallels to musical octaves. As a result, it is known as Newland’s Law of Octaves. The disadvantages of the octaves law are that it is only relevant up to Ca, and after Ca, every eighth element does not have attributes identical to the first. When Newland issued this legislation, there were only 56 elements in nature, but since then, many elements have been found. As a result, the remaining components and their attributes did not adhere to the law of octaves.
Mendeleev’s Periodic Table: In 1869, Russian scientist Dmitri Ivanovich Mendeleev developed a Periodic rule. He began his investigation with just 63 known elements. He discovered a link between the elements’ atomic masses and their physical and chemical qualities, as well as a periodic recurring trend with comparable physical and chemical attributes.
Vertical columns are referred to as ‘groups,’ while horizontal rows are referred to as ‘periods,’ in Mendeleev’s Periodic Table.
Mendeleev’s Periodic Table has the following advantages:
- Mendeleev’s Periodic table corrects various atomic masses of elements.
- There were many empty slots in Mendeleev’s Periodic table for additional element discovery. He called the hitherto unknown elements Eka-Boron, Eka-Aluminium, and Eka-silicon. He also anticipated their qualities as well as their atomic masses. These elements are now known as Sc, Ga, and Ge.
Mendeleev’s Periodic Table has the following advantages:
- Mendeleev was unable to accurately assign hydrogen’s location in the periodic table.
- Mendeleev’s periodic table assigned isotopes the same location as their elements, yet isotopes have distinct atomic masses.
- It has been observed that greater atomic mass elements are placed before lower atomic mass elements, for example, Co is placed before Ni.
Modern Periodic Table of Elements
In 1913, English physicist Henry Moseley proposed the current periodic law, which states:
“Physical and chemical properties of the elements are the periodic function of their atomic numbers”.
An element’s atomic mass is the total mass of the protons and neutrons present in a nucleus, whereas its atomic number is the total number of protons present in a nucleus. We know that the number of protons existing outside the nucleus equals the number of electrons. And the electron is the most significant factor in defining an element’s physical and chemical characteristics.

Read more about Reactions of Period 3 Elements and Their Oxides
Elements’ Positions in the Modern Periodic Table
There are 18 vertical columns, or 18 groups, and 7 horizontal rows, or periods.
Every period can denote the energy levels and the number of shells present in an element’s atom.The periodic table in its long form has a separate panel at the bottom. It comprises 14 elements from the sixth period known as lanthanoids and 14 elements from the seventh period known as actinoids. Elements are classified into four types or blocks based on their electron configuration. These are the s, p, d, and f blocks.
- The first period is made up of two elements: hydrogen and helium.
- The second phase contains eight elements, ranging from lithium to neon.
- The third period contains eight elements, ranging from sodium to argon.
- The fourth period has eighteen elements ranging from potassium to krypton.
- The fifth period has eighteen elements ranging from Rubidium to Xenon.
- The sixth period consists of thirty-two elements.
s-block elements are the first and second group elements with the electrical configuration ns1-2. p-blocks are the 13th and 18th group elements with the electrical configuration ns2np1-6.
(Both the s and p-block components are also referred to as representative elements.)
The elements of the third and twelfth groups are known as d-block or transition metals. (n-1) d1-10ns1-2 is their electrical configuration. Lanthanides and actinides are elements of the f-block or inner transition metals family, with the electronic configuration (n-2) f1-14(n-1) d0-1ns2. Noble gasses placed in group 8th are referred to as noble or inert gasses because its outermost S and P subshells are totally filled. As a result, they are exceedingly unresponsive.
Trends in the Modern Periodic Table
Valency: The valency of an element is obtained by the number of valence electrons present in the outermost shell of its atom. And the number of valence electrons is equal to the number of groups of the periodic table.
Atomic Size (radii): The atomic radii are defined as the distance between the nucleus’s center and the shell’s outermost electrons. When we travel from left to right in a period, the atomic radius reduces owing to an increase in effective nuclear charge (Zeff), while it grows due to an increase in shielding effect.
Ionic Radius (Size): The half distance between atomic ions in a crystal lattice is known as the ionic radius. The ionic radius grows as we proceed along a column or group. As well as declines from left to right throughout a row or period.
Energy of Ionization: Ionization energy is the amount of energy necessary to remove one electron from the outermost orbit of an isolated gaseous atom. The ionization energy of an element increases from left to right in a period and drops from top to bottom in a group.
Electronegativity: Electronegativity is the tendency of an atom in a molecule to attract the shared pair of electrons. Electronegativity rises from left to right over time and declines from top to bottom in a group.
References
- https://ncert.nic.in/ncerts/l/kech103.pdf
- https://www.sciencedirect.com/science/article/pii/B9780750633659500080
- https://www.frontiersin.org/articles/10.3389/fchem.2020.00813/full
- Image Sources: Wikipedia



